Hydrogen Production
A Guide to the Latest Advancements and Sustainable Methods
Why Hydrogen?
Hydrogen is the most abundant element in the universe and has the potential to revolutionize clean energy. It can be used in heavy industry, mobility fueling, industrial operations, and fuel cells to power everything from cars and trucks to buildings and entire cities, producing only water as a byproduct.
Methods Of Hydrogen Production
There are several methods of producing hydrogen on an industrial scale, each with their own pros and cons. We cover these methods in detail, including Steam Methane Reforming, Electrolysis of Water, Methane Pyrolysis, Photobiological and Photocatalytic Water Splitting, Fermentative Hydrogen Production, Thermochemical Cycles, Nuclear-Assisted Hydrogen Production, Coal Gasification, Natural Gas Reforming, Electrochemical Hydrogen Compression, and Biomass Gasification.
Sustainability and Environmental Impact
While hydrogen has the potential to be a clean and sustainable energy source, not all methods of hydrogen production are equally environmentally friendly. Explore the emissions and costs associated with each method, as well as the latest advancements in green hydrogen technology.
Safety and Economic Considerations
Hydrogen requires special handling and storage procedures. We discuss the safety concerns associated with hydrogen production and storage, as well as the economic considerations and government policies that are driving investment in this field.
Latest Innovations and Future Prospects
We cover the latest advancements in hydrogen production technology, including new materials, catalysts, and processes. We also examine the future prospects for hydrogen production, from its potential to power a hydrogen-based economy to the barriers that must be overcome to make this a reality.
Hydrogen Production Methods: A Comparison
Steam Methane Reforming
High hydrogen yield from methane
Requires high temperature steam
Produces carbon dioxide
Methane Pyrolysis
Heats methane to split carbon from hydrogen atoms
Powered by a portion of the hydrogen produced
Produces pure hydrogen and solid carbon

Electrolysis of Water
Uses electricity to split water into hydrogen and oxygen
Requires significant amounts of electricity
Produces pure hydrogen

Photobiological and Photocatalytic Water Splitting
Uses light and catalysts to split water
Mimics natural photosynthesis
Produces hydrogen and oxygen
Fermentative Hydrogen Production
Uses microorganisms to produce hydrogen from organic matter
Low energy input required
Produces hydrogen and carbon dioxide

Thermochemical Cycles
Uses heat and chemical reactions to split water
Requires high temperatures
Theoretically efficient but technically challenging

Nuclear-Assisted Hydrogen Production
Uses nuclear energy to power hydrogen production methods
Potential for large-scale, low-carbon hydrogen
Safety, economic, and public acceptance challenges

Coal Gasification
Converts coal into hydrogen, carbon monoxide and carbon dioxide
Requires high temperatures and pressures
Produces carbon dioxide emissions

Natural Gas Reforming
Steam methane reforming is currently the most common method
Requires natural gas or biogas feedstock
Well-established, large-scale technology

Electrochemical Hydrogen Compression
Uses electrolysis to compress hydrogen for storage
Requires electricity and produces heat
Theoretically efficient but still developing

Biomass Gasification
Converts biomass into hydrogen, carbon monoxide and carbon dioxide
Requires dry biomass feedstock and produces some emissions
Potentially carbon neutral and renewable
Questions about getting started with Hydrogen?
Drop us a line to schedule a free consultation!
This FAQ section covers common questions about hydrogen production, including methods of production, costs, safety concerns, environmental impact, latest innovations, future prospects, and government policies, drawing on a variety of sources for the most up-to-date information.
General FAQs
Hydrogen is produced on an industrial scale using various methods, including methane pyrolysis, steam methane reforming, electrolysis of water, and natural gas reforming.
The different methods of hydrogen production include methane pyrolysis, steam methane reforming, electrolysis of water, photobiological and photocatalytic water splitting, fermentative hydrogen production, and others. See a comparison of hydrogen production methods above.
The costs associated with hydrogen production vary depending on the method used, with some methods like electrolysis being more expensive than others like steam methane reforming.
The costs associated with hydrogen production vary depending on the method used, with some methods like electrolysis being more expensive than others like steam methane reforming.
Hydrogen can be produced at home for experiments using electrolysis kits, which split water into hydrogen and oxygen using electricity.
Different hydrogen production technologies have varying levels of efficiency, with some like photobiological and photocatalytic water splitting being less efficient than others like steam methane reforming with existing technologies.
The main barriers to scaling up hydrogen production include high costs, lack of infrastructure, and the need for greater technological advancements.
Environmental FAQs
Hydrogen production can be environmentally friendly if produced from renewable sources like wind or solar power or have efficient decarbonization methods such as methane pyrolysis, but some methods like coal gasification can have high emissions of carbon dioxide and other pollutants.
The future prospects for hydrogen production are promising, with the potential for hydrogen to be used as a clean energy source in transportation, power generation, and other applications as well as in hard-to-abate sectors.
The latest innovations in hydrogen production technology include advancements in catalysts and materials used in the production process, distributed and on-site hydrogen production methods, as well as new methods of hydrogen storage and transport.
The pros of different hydrogen production methods include efficiency, low emissions, and scalability, while the cons can include high costs, emissions, and safety concerns.
Different hydrogen production technologies have varying levels of efficiency, with some like photobiological and photocatalytic water splitting being less efficient than others like steam methane reforming with existing technologies.
Hydrogen Across The Globe FAQs
Around 10-11 million metric tons of hydrogen are produced globally each year, with the US accounting for around 10% of that total. Sources for up to date data include the International Energy Agency (IEA), U.S. Energy Information Administration (EIA), or industry reports from organizations such as the Hydrogen Council or the National Renewable Energy Laboratory (NREL).
Many companies are leading hydrogen production efforts along with Modern Hydrogen, including Toyota, Hyundai, Air Liquide, and others.
Many governments have policies and incentives to support hydrogen production, with some countries like Japan and South Korea investing heavily in developing hydrogen infrastructure and technologies. The United States provides hydrogen production credits through the Inflation Reduction Act; however, how those credits should be applied and who is eligible is still being debated.
Hydrogen production differs in various countries and regions based on factors like access to natural resources, government policies, and technological advancements.
Quick Links

Hydrogen production FAQ: methods, costs, safety, environment, innovation, government policies.
Have More Questions?
How long will it take you to decarbonization?
What’s the cost of decarbonization with hydrogen?
What is your CO2 intensity now, and how does that compare to using renewables or Modern Hydrogen?
"*" indicates required fields
Learn More About Hydrogen
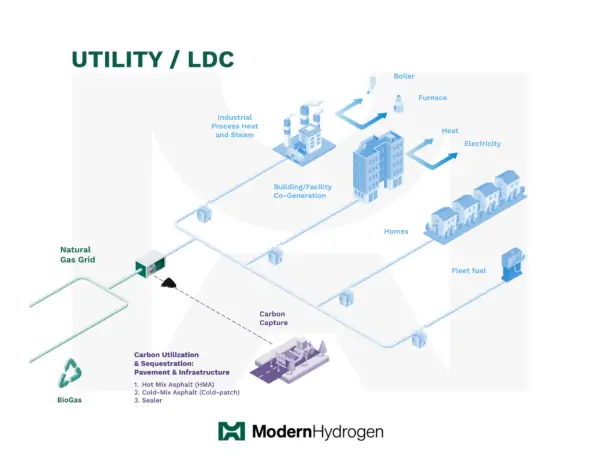
Utility Hydrogen

Turquoise Hydrogen

Methane Pyrolysis